The story of hypercare
We are ready to launch a new product
Tecvayli, a pioneering BCMAxCD3 bispecific large molecule drug, addresses unmet needs in Multiple Myeloma treatment.
Read more
We are ready to launch a new product
Tecvayli, a pioneering BCMAxCD3 bispecific large molecule drug, addresses unmet needs in Multiple Myeloma treatment.
Tecvayli, launched in October 2022 in Europe and November 2022 in the U.S., provides a valuable first mover advantage in Multiple Myeloma, making it a significant player in advancing treatment options for this complex condition. Tecvayli is complementary to Darzalex, Talvey and Carvykti, enhancing our portfolio while offering a comprehensive approach to Multiple Myeloma treatment.
We faced challenges
In 4Q ‘22, we encountered production challenges resulting in the rejection of 4 batches in total.
Read more
We faced challenges
In 4Q ‘22, we encountered production challenges resulting in the rejection of 4 batches in total.
2 CD3 upstream batches at Leiden and 2 downstream batches at Cork were terminated during 4Q ‘22. This resulted in reduced safety stock levels at a time we were preparing for global launches while experiencing dynamic commercial and clinical forecast signals due to the overall positive performance of the drug.
Hypercare for flawless execution and success
Hypercare was installed in February 2023 to ensure flawless execution and success of our product.
Read more
Hypercare for flawless execution and success
Hypercare was installed in February 2023 to ensure flawless execution and success of our product.
The key objectives of Hypercare encompass proactive monitoring of End-to-End business operations and performance, a thorough review of risk mitigation and a structured communication plan with monthly updates to the Janssen Supply Chain (JSC) Leadership Team.
Check out our results
Our supply chain has made a fantastic shift from being at-risk to achieving safety stock target at API intermediate level, enabling a stabilized supply chain and entry to new markets.
Read more
Check out our results
Our supply chain has made a fantastic shift from being at-risk to achieving safety stock target at API intermediate level, enabling a stabilized supply chain and entry to new markets.
In both Upstream and Downstream Processing, we've seen impressive performances meeting the agreed global commercial and clinical upside supply target, by achieving greater than 80% reduction in Quality Investigations.
Moreover, we're celebrating over 37 successful launches within the first 12 months, marking a remarkable milestone in our overall journey.
37
+
Successful launches
37
+
Successful launches
Achievements
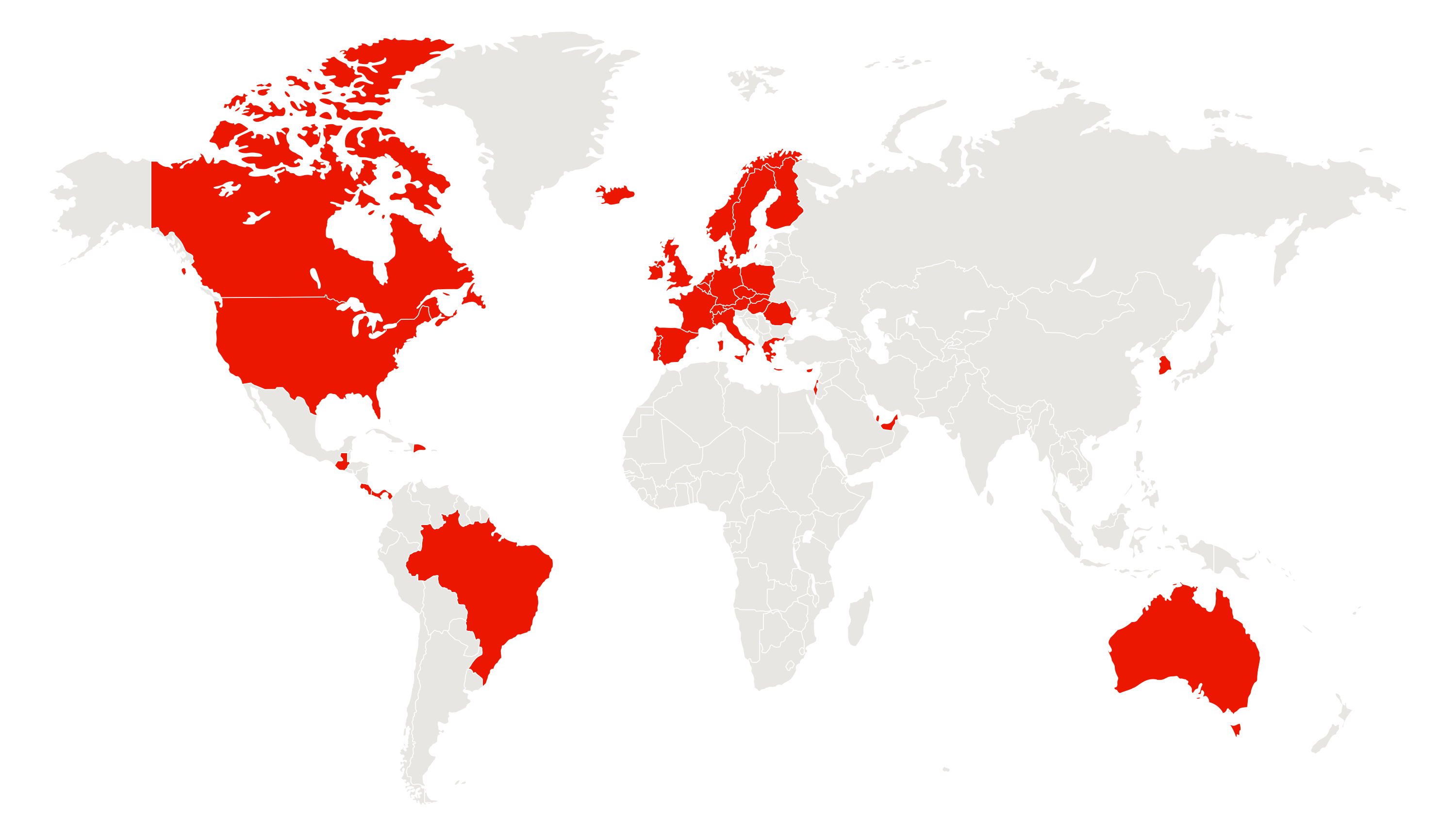
37+ successful launches
Thanks to the Hypercare efforts we were able to successfully launchTecvayli in more than 37 markets. This is a testament to our capability to effectively expand product reach and provide access to valuable solutions for a broader patient population.
37+ successful launches
Thanks to the Hypercare efforts we were able to bring Tecvayli in more than 37 markets.
This is a testament to our capability to effectively expand product reach, providing valuable solutions to a broader audience.
New CD3 Working Cell Bank qualification
The on-track qualification of a new CD3 Working Cell Bank marks a significant milestone, ensuring a seamless re-supply of CD3 upstream intermediate. This achievement includes the successful completion of five CD3 batches in Leiden and three Downstream batches in Cork.
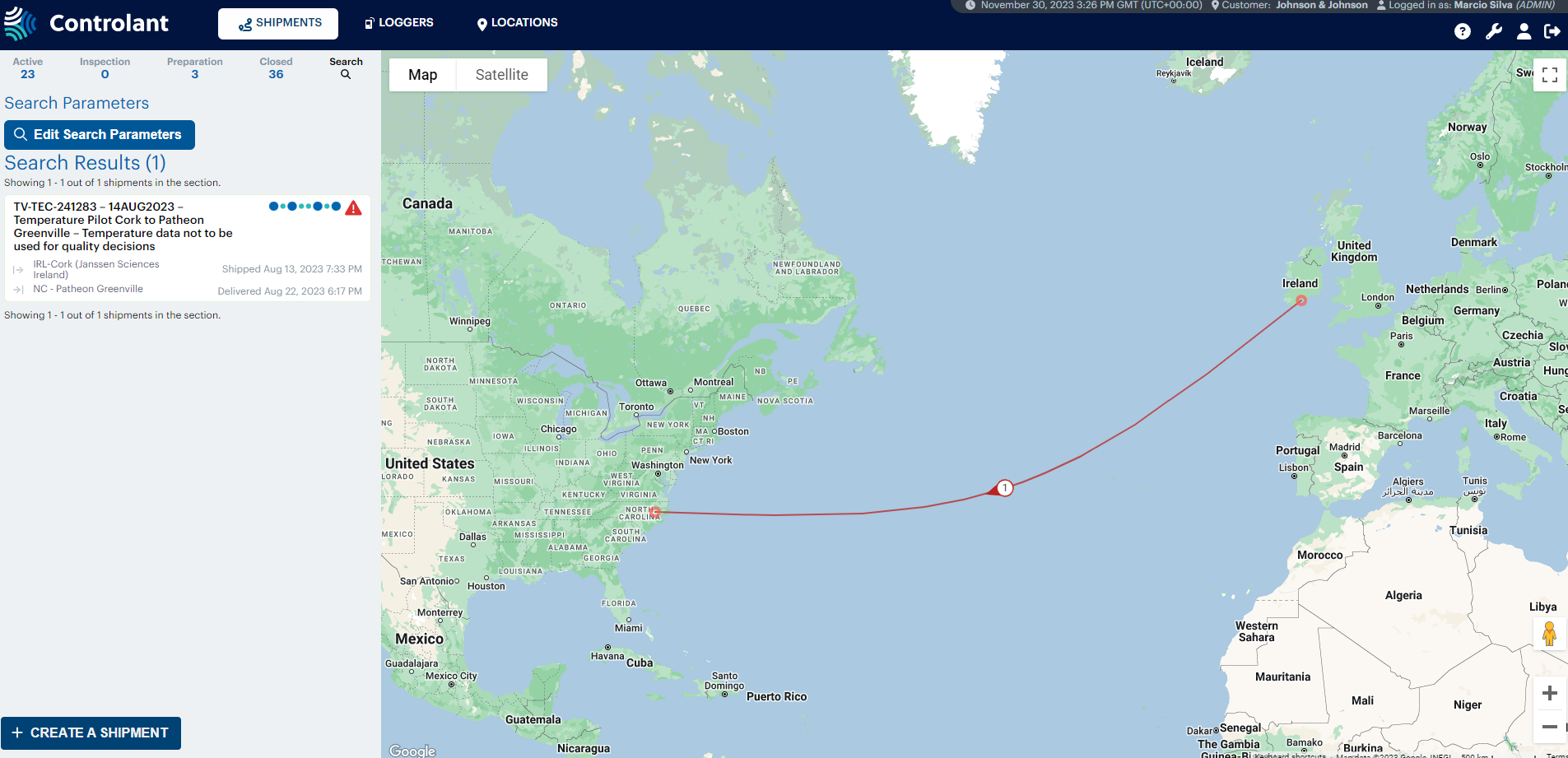
New qualified shipping lanes
The shipping process of frozen drug substance from Cork to Patheon has undergone significant enhancements to optimize efficiency and flexibility within our E2E supply chain.
Read more
New qualified shipping lanes
The shipping process of frozen drug substance from Cork to Patheon has undergone significant enhancements to optimize efficiency and flexibility within our E2E supply chain.
Two new shipping lanes, connecting Cork and Patheon are now live, utilizing FedEx and DHL. The 2023 performance analysis of this route revealed an impressive reduction in average lead time by 2 days with DHL compared to FedEx.
The team also successfully completed a Controlant device pilot. This real-time temperature monitoring device allows us to GPS track the shipments and actively monitor and control temperature conditions, ensuring that our pharmaceuticals reach their destination in optimal condition.
These optimizations not only have de-risked international shipments of a high value intermediate and improved the E2E cycle time for Tecvayli, but can also be leveraged by other brands utilizing the same flow.

Labeling and packaging capacity optimization
Strategic initiatives have led to the optimization of labeling and packaging capacity at Packaging Coordinators Inc (PCI) Rockford.
Read more
Labeling and packaging capacity optimization
Strategic initiatives have led to the optimization of labeling and packaging capacity at Packaging Coordinators Inc (PCI) Rockford.
The qualification of a semi-automated packaging line has increased efficiency for higher volume orders, and implementation of an additional shared manual packaging line is temporarily doubling the capacity for Tecvayli and Talvey at PCI Rockford. Additionally, the qualification of an alternative Tier II raw material supplier for labels and the activation of a second source for cartons have significantly boosted resilience.

IT and Automation enhancement
By automating the purchase order (PO) creation in Europe2 (E2), the team removed the manual efforts to mirror data from the Sustain system (IT Interface Sustain/E2).
Read more
IT and Automation enhancement
By automating the purchase order (PO) creation in Europe2 (E2), the team removed the manual efforts to mirror data from the Sustain system (IT Interface Sustain/E2).
In addition, Europe2 to eLIMS batch linking automation was implemented, resulting in elimination of manual steps previously required for the eLIMS team to link every batch shipped from PCI to EDC. This IT solution enables quicker and more seamless quality release of products, while eliminating non-value-added manual activities across more than 100 batches per year.
These Automation enhancements can be leveraged by other brands utilizing the same flow.

Doubling production capacity with additional bioreactors in Leiden
2 additional 2kL bioreactors were successfully implemented, resulting in doubling our production capacity.
Read more
Doubling production capacity with additional bioreactors in Leiden
2 additional 2kL bioreactors were successfully implemented, resulting in doubling our production capacity.
This marks a triumph of progress and resulted in 3.5-month stock coverage of CD3 upstream intermediate, showcasing our operational efficiency. The Hypercare approach at the Leiden site was characterized by proactive End-to-End batch monitoring, rapid responses, strengthened alignment, and a determination to leave no stone unturned in risk mitigation. Hypercare gave us the passionate focus to drive our processes forward and we are taking these learnings into the foundation of our business, cascading benefits across all our products.

Strong performance in Quality and Operations in Cork
The implementation of the Hypercare process into our day-to-day operation has resulted in a new way of operating for the BU and Cork site.
Read more
Strong performance in Quality and Operations in Cork
The implementation of the Hypercare process into our day-to-day operation has resulted in a new way of operating for the BU and Cork site.
Enabling a far greater focus on the “operation floor” – helping to ensure that “barriers” to flow are quickly removed and the team, running the process, are getting the support they need, when they need. Hypercare is driving better prioritization and focus on the manufacturing process. The Hypercare process has also allowed us to proactively identify risks and implement mitigations. In 2024 this will no longer be the Hypercare model – it will be our standard operating model.

Fostering partnership with External Drug Product Manufacturing Site
By successfully engaging with our external drug product manufacturing partner, Patheon Greenville, the performance moved to the next level, achieving excellent results.
Read more
Fostering partnership with External Drug Product Manufacturing Site
By successfully engaging with our external drug product manufacturing partner, Patheon Greenville, the performance moved to the next level, achieving excellent results.
Efficiency improvement strategies identified during PES Value Creation sessions, combined with ongoing vigilant monitoring, led to a remarkable accomplishment of 100% On-Time In-Full (OTIF) in 2023 for drug product from Patheon Greenville.

Improving transportation efficiency
In addition to the Cork to Patheon GV route, the team focused on improving transportation efficiency of finished goods distribution from our Packaging Coordinators Inc (PCI) site to the European Distribution Center (EDC).
Read more
Improving transportation efficiency
In addition to the Cork to Patheon GV route, the team focused on improving transportation efficiency of finished goods distribution from our Packaging Coordinators Inc (PCI) site to the European Distribution Center (EDC).
Through the validation of a new lane, the team successfully reduced transit time from PCI to EDC by 3 days. This validation not only shortened transit times but also allowed the introduction of a more frequent (twice a week) pick-up schedule, enhancing our ability to efficiently serve patients.
These optimizations not only improved the E2E cycle time for Tecvayli, but can also be leveraged by other brands utilizing the same flow.
Meet the team
Meet our phenomenal team, a group of dedicated professionals operating in different areas across our dynamic E2E supply chain. Each member is an expert, chosen for their profound expertise, direct connectivity across various sites and processes, and End-to-End mastery of our complex supply chain. The creativity, ingenuity, and collaboration across the team ensures harmonized communication and decision making. It is the extraordinary efforts and contributions across the core and extended teams that has made and continues to make Tecvayli Hypercare a tremendous success.
All this while working remote, proving that virtual teams can drive innovative solutions, and collaboration transcends boundaries. Get acquainted with the minds powering our Hypercare Team by clicking on their photo to learn more about the skills and experiences of each core team member.
‘‘
A final thanks to those who made it happen!’’
Herewith, we want to acknowledge the contributions and extend our gratitude to the following extended team members and other functional groups: John McNally, Stephanie Gossett, Snehal Patil, Kevin Muthurania, Rada Dubashinsky, the Value Chain Team, Cork cross functional teams (Alex Browne, Caroline Lynch, Jessica Barrett, Colm O'Donovan in Operations, Annmarie Barton in QA and the Site planning Oonagh Brennan), Leiden cross functional teams (Manuella Wilts, Suzanne Seegers-twomey, Jan-Dirk Vooijs, Jimmy Ajodhia, Annemiek Takens, Jurriaan van der Velden, Wesley van den Berg, Samantha Ong, Koen van Kuik, Martijn Bastiaans), Cold Chain Operation teams, Site Planning (internal and external), and Partnership & External Supply team.

Todd Gibson
Tecvayli VCL and Hypercare Team Sponsor
Meet Todd
Todd Gibson
Tecvayli VCL and Hypercare Team Sponsor
I provide sponsorship and leadership support to the team with a focus on on-time delivery and establishing effective risk mitigation plans. My motivation comes from working with a dedicated team to ensure Tecvayli is supplied on time to every patient in need. A personal highlight is witnessing the engagement and collaboration across the entire team, which has resulted in phenomenal performance enabling us to overcome challenges and deliver Tecvayli to patients across the globe.

Alessandra Catalani
Hypercare Team Lead
Meet Alessandra
Alessandra Catalani
Hypercare Team Lead
I lead the team focusing on E2E product flowless execution and on time delivery. My motivation comes from a clear consciousness: the Hypercare drives to a reliable supply chain which directly impacts our patients’ lives, every single day. A personal highlight is seeing a trusted and integrated team E2E with a natural innovative attitude towards the boundless research of excellence in execution.

Michael Sisko
E2E Planning Manager
Meet Michael
Michael Sisko
E2E Planning Manager
I am leading the E2E planning activities, and I am motivated by the mission to ensure delivery to patients in need and the success of the Tecvayli brand while optimizing the E2E Supply Chain.
A personal highlight is being a member of this committed cross-functional group who have been leveraging an E2E approach to driving outstanding performance and results, and the impressive insights gained into the critical work of the great teams enabling these results behind the scenes.

Niloufar Sadeghipour
Hypercare Site Lead in Leiden
Meet Niloufar
Niloufar Sadeghipour
Hypercare Site Lead in Leiden
I am leading CD3 Hypercare activities for the Leiden site. My motivation is to ensure consistent delivery of this life-saving medication for our patients. A personal highlight for me in the Hypercare project is witnessing the End-to-End approach, cross sites collaboration and seamless communication that creates a bridge from shop floor to platform Tecvayli. It is a project that instils purpose, making every action important and inspire us to integrate an End-to-End approach into our base business.

Ronan Hayes
Project Lead for Hypercare in Cork
Meet Ronan
Ronan Hayes
Project Lead for Hypercare in Cork
I am responsible for enabling and delivering the Tecvayli Hypercare process on site. My motivation is simple but profound - Hypercare is pivotal in delivering a crucial, ground-breaking product to our patients. A personal highlight is seeing the transformational impact of the Tecvayli Hypercare process, which fosters a more connected, engaged, and collaborative cross-functional team and fundamentally changes our operational dynamics.

Anik Warner
Cold Chain Operations Lead
Meet Anik
Anik Warner
Cold Chain Operations Lead
I oversee Cold Chain Operations, focusing on enhancing Hypercare shipment support, carrier management, and transportation process improvements. My motivation is rooted in a commitment to ensuring flawless execution in every shipment to meet customer demands. A personal highlight has been observing the improvements in lead time performance, particularly through successful lane optimization initiatives.

Kelly Spence
PES Site Lead for Patheon
Meet Kelly
Kelly Spence
PES Site Lead for Patheon
I am site lead PES at Patheon, and my role revolves around ensuring the successful production and on-time shipment of Tecvayli from Patheon Greenville to PCI Rockford. I am driven by a patient-first mentality, striving to connect operations end-to-end to ensure the timely availability of our products. A personal highlight for me is the genuine connectivity achieved through the Hypercare forum, where collaboration and teamwork have been truly exceptional!

Kristine Laswell
NPI Planner
Meet Kristine
Kristine Laswell
NPI Planner
I play a key role as an NPI Planner in the Hypercare Team. My mission revolves around providing support and feedback to the E2E Planner and PES, ensuring a smooth flow in our operations. My drive is fueled by the determination to guarantee our product reaches patients right on time. For me, the personal highlight in the Hypercare project is the collaborative spirit, working together towards a common goal of delivering on time to our patients.

Sahlee Young
PES Site Lead for PCI Rockford
Meet Sahlee
Sahlee Young
PES Site Lead for PCI Rockford
I am the PES Site Lead for PCI Rockford. My role is centred on managing PCI activities, focusing on the efficient packaging and shipment of Tecvayli and Talvey. I am driven by the opportunity to contribute to a team that delivers innovative, life-changing medicines to patients. A personal highlight for me in this project has been witnessing the extraordinary collaboration across various departments, all committed to the unified goal of delivering Tecvayli and Talvey to those in need.